Quick, what’s the first thing you think of when you hear the word Rum? If you are picturing a pirate then you are like most people. Now what’s that tune you are hearing in your head? If you are hearing “Yo-Ho-Ho and a bottle of Rum” then you passed trivia test number two. Now if you are shedding a tear as you think about the scene from Pirates of the Caribbean: Curse of the Black Pearl when Elizabeth Swan lit Captain Jack Sparrows stash of rum on fire, then you are a true fan of rum and will enjoy this science lesson.
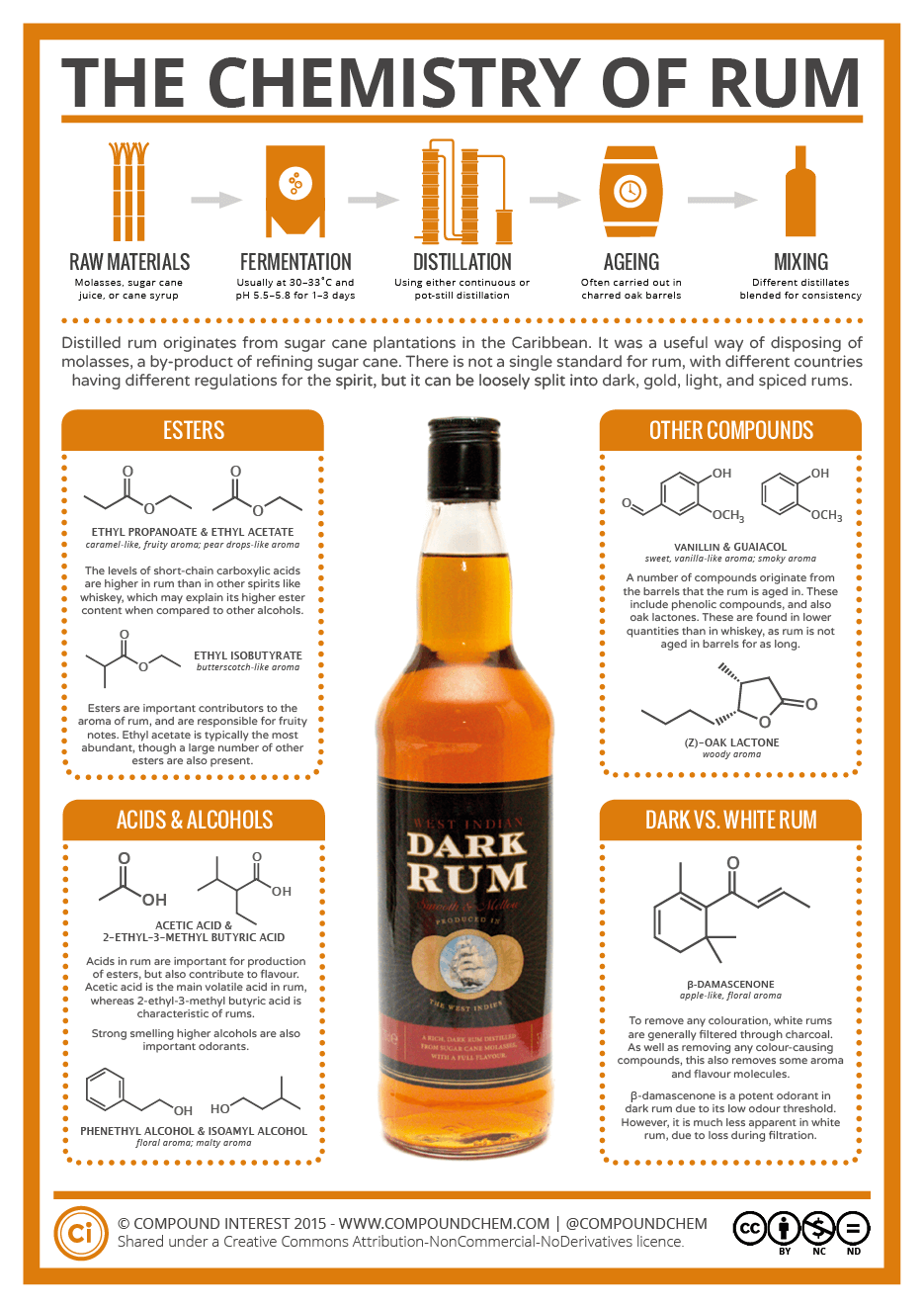
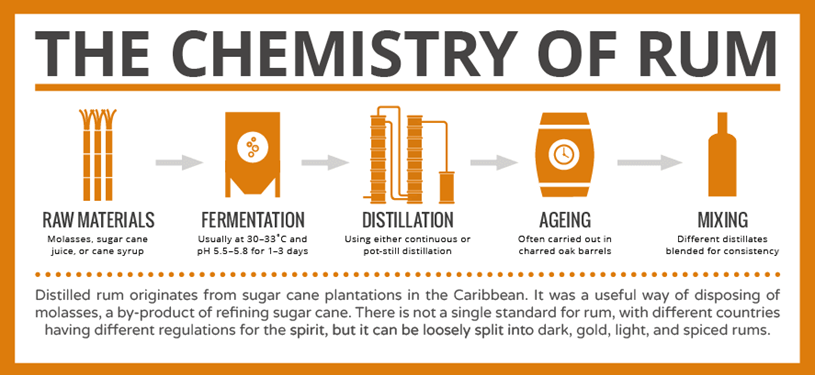
Rum originates from the process that gives us sugar. Sugar cane has to be processed to produce sugar, and this processing produces a syrupy fluid known as molasses. Rum is a distilled alcoholic beverage made from fermented molasses.
Stay Informed: Sign up here for our Distillery Trail free email newsletter and be the first to get all the latest news, trends, job listings and events in your inbox.
So, what is it that makes rum, on a chemical level? In terms of the aroma, ester compounds have a big part to play. You might have come across esters in chemistry class – they’re relatively easy to make, by reacting an organic acid with an alcohol, and are characterized by their range of different aromas. Some smell fruity, some smell medicinal, some smell like glue.
There are a whole range of ester compounds found in rum; they’re often the dominant class of organic compounds found in the spirit. The range of esters adds the fruitiness to rum’s aroma; particularly important contributors are ethyl propanoate which contributes a caramel-like, fruity aroma, and ethyl isobutyrate which has a butterscotch-like aroma. Rum has a higher short-chain carboxylic acid content compared to other spirits, which may also help explain why its ester content is higher than other alcohols.
Related Stories
The Chemistry Behind The Whisky We Love So Much [Infographic]
The Chemistry Behind the Gin We Love So Much [Infographic]
The Chemistry Behind That Starbucks You Can’t Live Without [Infographic]
What is Rum?
It’s not just the esters that contribute to the aroma and flavor, however. The acids that help to form the esters can, themselves, have an impact. Additionally, higher alcohols (that is, those with more carbons than ethanol) also contribute. Phenethyl alcohol adds a floral aroma, and is actually also found in the aroma of roses and several other flowers. Isoamyl alcohol adds a more malty note.
The afore-mentioned compounds derive from compounds already present in rum. However, during the aging process, compounds from the barrels the rum is contained in can also end up in the mix. These include a number of phenolic compounds, which can impart medicinal and smoky notes. They also include compounds like vanillin, the major flavor and aroma component of vanilla. Oak lactones are also found in rum, though to a lesser extent than in whiskeys which tend to be aged for longer.
Click here to view the full size Chemistry of Rum infographic.